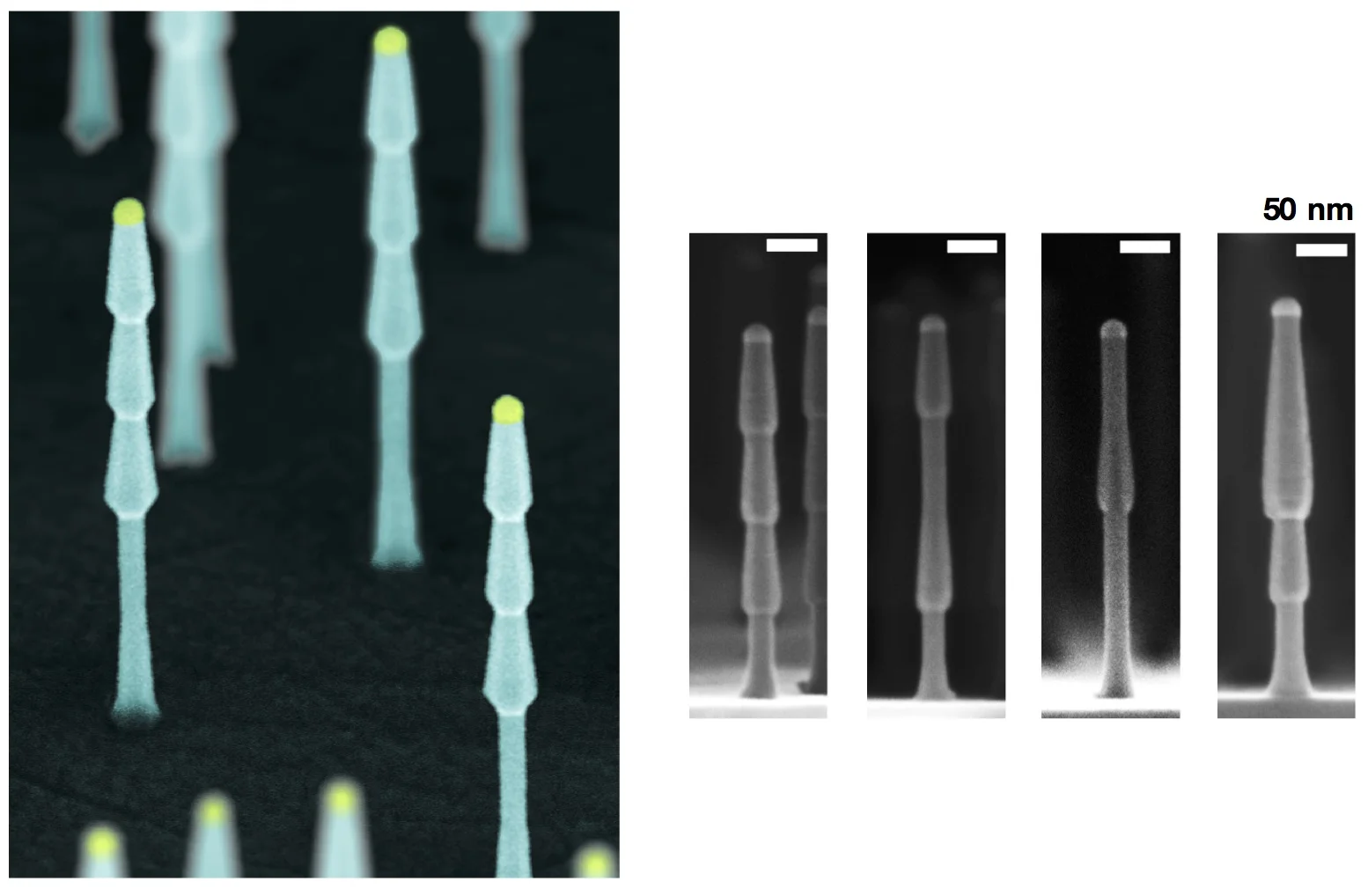
We specialize in the synthesis, understanding, and large-scale deployment of nanoscale materials and devices to enable new electronic, photonic, and energy technologies.
Image from: I. R. Musin, D. S. Boyuk, M. A. Filler, Surface Chemistry Controlled Diameter-Modulated Semiconductor Nanowire Superstructures, J. Vac. Sci. Technol. B. 31 020603 (2013).
LATEST NEWS
Find all the latest information regarding publications, presentations, awards, and more.
LEARN MORE
Explore our publications appearing in a range of scientific journals.
Prof. Filler
Dr. Michael A. Filler is a Professor and the Traylor Faculty Fellow in the School of Chemical & Biomolecular Engineering at the Georgia Institute of Technology.